Prebiotically plausible organocatalysts enabling a selective photoredox α-alkylation of aldehydes on the Early Earth
A.C. Closs et.al. 2020 Chem. Eur. J. https://doi.org/10.1002/chem.202001514
31.03.2020
A.C. Closs, E. Fuks, M. Bechtel and Trapp, O
Chem. Eur. J. https://doi.org/10.1002/chem.202001514
Abstract
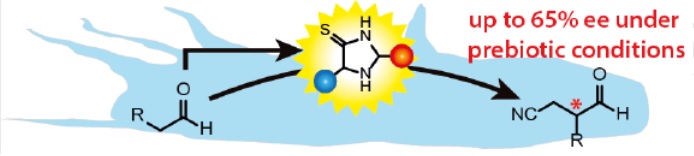 Organocatalysis is a powerful approach to extend and (enantio‐) selectively modify molecular structures, as those comprising the prebiotic feedstock. Adapting this concept to the Early Earth scenario offers a promising solution to explain their evolution into a complex homochiral world. In this work, we present a class of imidazolidine‐4‐thione (photoredox‐) organocatalysts, easily accessible from simple molecules available on an Early Earth under highly plausible prebiotic reaction conditions. These imidazolidine‐4‐thiones are readily formed from mixtures of aldehydes or ketones in presence of ammonia, cyanides and hydrogen sulphide in high selectivity and distinct preference for individual compounds of the resulting catalyst library. These organocatalysts successfully enable the challenging enantioselective α‐alkylation of aldehydes under prebiotic conditions and show activities that correlate with the selectivity of their formation. Furthermore, the crystallization of single catalysts as conglomerates opens the pathway for symmetry breaking.
Organocatalysis is a powerful approach to extend and (enantio‐) selectively modify molecular structures, as those comprising the prebiotic feedstock. Adapting this concept to the Early Earth scenario offers a promising solution to explain their evolution into a complex homochiral world. In this work, we present a class of imidazolidine‐4‐thione (photoredox‐) organocatalysts, easily accessible from simple molecules available on an Early Earth under highly plausible prebiotic reaction conditions. These imidazolidine‐4‐thiones are readily formed from mixtures of aldehydes or ketones in presence of ammonia, cyanides and hydrogen sulphide in high selectivity and distinct preference for individual compounds of the resulting catalyst library. These organocatalysts successfully enable the challenging enantioselective α‐alkylation of aldehydes under prebiotic conditions and show activities that correlate with the selectivity of their formation. Furthermore, the crystallization of single catalysts as conglomerates opens the pathway for symmetry breaking.

